CO2 capture technology is vital for reducing greenhouse gas emissions. But what happens when the chemicals used in this process wear out or degrade? Scientists have been studying how to rejuvenate these chemicals through a method called thermal reclaiming. This research focuses on ethanolamine (MEA), a widely used solvent for capturing CO2, to understand how to improve its reuse and reduce waste.
Thermal reclaiming is like giving old solvents a makeover. Over time, the chemicals used in CO2 capture degrade due to heat, impurities, and chemical reactions. These changes can make them less effective. Thermal reclaiming heats the degraded solvent under controlled conditions to remove harmful byproducts and recover usable chemicals.
Why Focus on Ethanolamine (MEA)?
Ethanolamine (MEA) is one of the most common solvents for CO₂ capture due to its effectiveness and relatively low cost. However, it is prone to degradation, resulting in the formation of numerous byproducts. Researchers selected MEA for this study to gain a deeper understanding of its behavior under reclaiming conditions and to explore methods for its efficient reuse. This work not only advances knowledge about MEA but also lays the groundwork for designing methodologies to test other solvents, as will be undertaken with CESAR1 in AURORA this year, building on the foundation established in this study.
How Does Degradation Affect the Process?
Degradation makes the MEA solvent less effective at capturing CO₂ and introduces impurities that can harm equipment and increase costs. These byproducts fall into three categories:
- Heat Stable Salts (HSS): Non-volatile compounds that stay in the sludge.
- Volatile Compounds: Substances like ammonia and small aldehydes that may escape into the air.
- Complex Degradation Products: Compounds that are harder to remove and may affect the solvent’s performance.
What Did the Scientists Do?
The researchers tested thermal reclaiming on both fresh and degraded MEA under various conditions of temperature and pressure. They used sophisticated tools to analyze the reclaimed solvent and the leftover sludge. They also conducted simpler lab tests to simulate how different chemicals behave during the process.
Key Findings
- Efficiency of Reclaiming: Fresh MEA was easier to clean and recover compared to degraded MEA.
- Byproduct Behavior: Some harmful byproducts, like certain amides, were reduced during the process. However, new byproducts also formed, highlighting the need to optimize conditions.
- Alkaline Hydrolysis: Adding a strong base helped break down some stubborn compounds, but this method was more effective in lab tests than in real reclaiming scenarios.
- Environmental Concerns: Some volatile byproducts, like secondary amines, may escape during reclaiming. These compounds could form harmful substances like nitrosamines, emphasizing the need for strict emission controls.
Why Does This Matter?
Improving the efficiency of thermal reclaiming means less waste, lower costs, and better environmental outcomes. It also ensures that CO2 capture remains a viable tool in the fight against climate change.
The Authors
- SINTEF Industry, NO-7465 Trondheim, Norway: Vanja Buvik, Andreas Grimstvedt, Kai Vernstad, Merete Wiig;
- Department of Chemical Engineering, Norwegian University of Science and Technology (NTNU), N-7497- Trondheim, Norway: Hanna K. Knuutila;
- Aker Carbon Capture: Ricardo Wanderley.
Conclusion
Thermal reclaiming is a promising way to recycle the chemicals used in CO2 capture. While challenges remain, studies like this one provide valuable insights into making the process more effective and sustainable. As we continue to refine these methods, we get closer to a cleaner and greener future.
Publication – Development of a Validated Rate-Based Model
Abstract In this work, we developed a new e-NRTL thermodynamic framework for CO2 absorption in aqueous mixtures of 2-amino-2-methyl-1-propanol (AMP) and piperazine (PZ) in Aspen Plus. The e-NRTL AMP/PZ/H2O/CO2 model was fitted on experimental data covering a range of AMP concentration from 12 to 48 mass % and PZ concentration…
Publication – Pilot-scale CO2 capture in a cement plant with CESAR1
Abstract Carbon capture from hard-to-abate industries is essential. This study investigates the effect of stripper pressure on the performance of amine-based CO2 capture from cement flue gas, using the CESAR1 solvent. Through a rigorous data filtering and binning methodology, experimental results were systematically categorized, enabling a precise evaluation of how…
New Publication: Aerosol Modeling in CO2 absorption using CESAR1
We are proud to announce that Hallvard F. Svendsen, Hanna K. Knuutila, Ardi Hartono, Maxime Francois and Diego Morlando at NTNU, together with external collaborators Peter Moser (RWE) and Georg Wiechers (RWE) have published a new article presenting results from the AURORA and SCOPE projects.The publication introduces a new class-based…
Thermodynamic Properties of CO₂ Absorption in CESAR1 — Essential Data for Better Process Modelling
We are proud to announce a new scientific publication from the AURORA project, authored by Diego Morlando, Ardi Hartono and Hanna K. Knuutila, published in Carbon Capture Science & Technology. This important work provides extensive experimental data on the thermodynamic properties of CESAR1, a key solvent blend for industrial carbon…
Journal Publication – In-Depth Study of CESAR1 Solvent Degradation Under CO₂ Capture Conditions
A new scientific publication based on research from the AURORA project has just been released in the journal Industrial & Engineering Chemistry Research. This study delivers an in-depth analysis of the degradation behaviour of the CESAR1 solvent —a popular choice for solvent-based post-combustion CO₂ capture.This work, developed under the AURORA…
Conference publication – GHGT-17: Viscosity and Density data for the CESAR1 solvent
Abstract Global warming is a major issue that needs to be addressed and limited. The CESAR1 solvent blend has a high potential for becoming a commonly employed, commercial solvent system. In this work, the viscosity and density data for aqueous CO2 loaded CESAR1 (26.6 wt.% AMP 12.8 wt.% PZ) solution…
Conference publication – GHGT-17: “Storage potential evaluation of eastern Mediterranean area as final step of the full chainassessment”
The final step in capturing and storing carbon dioxide (CO₂) emissions is geological storage, where CO₂ is injected deep underground into carefully chosen locations. These locations could be natural formations like saline aquifers (underground reservoirs filled with salty water) or empty oil and gas fields.This work, part of the AURORA…
Understanding Solvent Degradation in CO₂ Capture – CESAR1 Solvent Degradation in Pilot and Laboratory Scale
The fight against climate change requires innovative solutions, and one promising method is CO₂ capture and storage (CCS). CCS involves capturing carbon dioxide from industrial emissions before it reaches the atmosphere. At the heart of this process are specialized chemical solvents, such as CESAR1, which absorb CO₂ from flue gases.While…
Closing Knowledge Gaps – Density and Viscosity of Unloaded and CO2-loaded Aqueous AMP-PZ blends
AURORA’s latest scientific journal publication provides experimental density and viscosity data on different unloaded and CO2-loaded aqueous blends of 2-amino-2-methyl-1-propanol (AMP) and piperazine (PZ) used for absorption-based CO2 capture. The paper also provides correlations for density and viscosity suitable for various modelling works.In our previous review article, we identified knowledge…
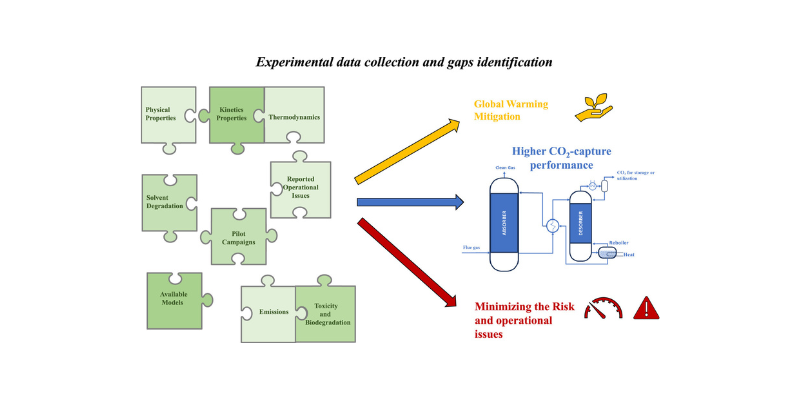
Unlocking New Potential of CESAR1-based chemical absorption Technology: Available data and knowledge gaps of the CESAR1 solvent system
AURORA latest review paper, developed in collaboration with researchers from SINTEF and NTNU, provides a comprehensive analysis of the CESAR1 solvent system. It collects and evaluates existing experimental data, highlights knowledge gaps, and outlines the necessary next steps in research to optimize the use of CESAR1 for CO₂ capture.In the…