A chemical mixture called CESAR1 is being widely studied as a solvent to capture carbon dioxide (CO₂) from industrial emissions (like power plants). CESAR1 is made up of two chemicals: AMP (2-amino-2-methyl propanol) and PZ (piperazine). It’s more stable and degrades less over time compared to another common solvent called MEA (monoethanolamine), even when exposed to heat, oxygen, and the tough conditions of CO₂ capture plants.
However, to safely use CESAR1 on a large scale, scientists need to fully understand how it breaks down (degrades) over time. Even though it degrades less than other solvents, some of the resulting breakdown products could impact the environment, plant safety, or nearby communities.
In this work, researchers are closely studying how and why CESAR1 degrades under realistic industrial conditions. By running experiments under heat and oxygen exposure, they are comparing lab results with real-world samples taken from CO₂ capture plants.
Recent research has already identified and measured over 30 specific CESAR1 degradation products—these include compounds like acetone and formaldehyde, which are chemicals that can form as the solvent breaks down. The research also measures the amounts of the two main solvent components, AMP and PZ, in used CESAR1 samples. A special test called total nitrogen (TN) analysis is also used to make sure no nitrogen-based compounds are missed in the breakdown process.
So far, a sample of CESAR1 taken from a test facility in Norway (the Technology Centre Mongstad, TCM) has been analyzed. This sample revealed the presence of many known degradation compounds, but most were found at very low levels. Overall, this detailed work is helping scientists ensure that CESAR1 can be safely used for large-scale CO₂ capture without unforeseen risks.
Key Terms Simplified
- Solvent: A liquid chemical that absorbs CO₂ from gases emitted by industrial plants.
- Degradation: When a chemical breaks down into smaller, often less stable parts due to heat, oxygen, or other stressors.
- Post-Combustion CO₂ Capture: A technology that removes CO₂ from flue gases after fuel (like coal or gas) is burned.
- AMP (2-amino-2-methyl propanol) and PZ (piperazine): The two chemicals that make up CESAR1 and help it capture CO₂.
- Degradation Products: New chemicals formed when CESAR1 breaks down. Some can be harmful or need careful monitoring.
- LC-MSMS: A very precise laboratory technique used to identify and measure tiny amounts of chemicals in a sample.
- Total Nitrogen (TN) Analysis: A test to see if all nitrogen-containing compounds are accounted for in a degraded solvent sample.
This research is uncovering exactly how CESAR1 behaves over time in CO₂ capture systems. By identifying its breakdown products, scientists can ensure it’s safe, reliable, and ready for use in reducing CO₂ emissions on an industrial scale.
Understanding Solvent Degradation in CO₂ Capture – CESAR1 Solvent Degradation in Pilot and Laboratory Scale
The fight against climate change requires innovative solutions, and one promising method is CO₂ capture and storage (CCS). CCS involves capturing carbon dioxide from industrial emissions before it reaches the atmosphere. At the heart of this process are specialized chemical solvents, such as CESAR1, which absorb CO₂ from flue gases.While…
Turning Waste Into Opportunity: Thermal Reclamation Chemistry of Common Amine Solvents
CO2 capture technology is vital for reducing greenhouse gas emissions. But what happens when the chemicals used in this process wear out or degrade? Scientists have been studying how to rejuvenate these chemicals through a method called thermal reclaiming. This research focuses on ethanolamine (MEA), a widely used solvent for…
Closing Knowledge Gaps – Density and Viscosity of Unloaded and CO2-loaded Aqueous AMP-PZ blends
AURORA’s latest scientific journal publication provides experimental density and viscosity data on different unloaded and CO2-loaded aqueous blends of 2-amino-2-methyl-1-propanol (AMP) and piperazine (PZ) used for absorption-based CO2 capture. The paper also provides correlations for density and viscosity suitable for various modelling works.In our previous review article, we identified knowledge…
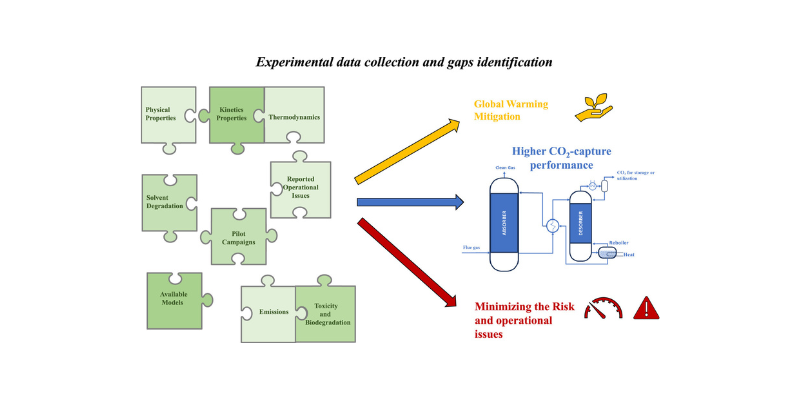
Unlocking New Potential of CESAR1-based chemical absorption Technology: Available data and knowledge gaps of the CESAR1 solvent system
AURORA latest review paper, developed in collaboration with researchers from SINTEF and NTNU, provides a comprehensive analysis of the CESAR1 solvent system. It collects and evaluates existing experimental data, highlights knowledge gaps, and outlines the necessary next steps in research to optimize the use of CESAR1 for CO₂ capture.In the…
Conference publication – Optimal Control of Industrial Solvent-Based CO2 Capture Plants Conference publication
This publication, prepared by our project partners Cybernetica and SINTEF Industry, is a proceeding from the 34th European Symposium on Computer Aided Process Engineering and the 15th International Symposium on Process Systems Engineering (ESCAPE34/PSE24), held in Florence, Italy, from June 2-6, 2024.Researchers have developed and tested advanced methods to control…